Selected Publications
Whiteley AT, Eaglesham JB, de Oliveira Mann CC, Morehouse BR, Lowey B, Nieminen EA, Danilchanka O, King DS, Lee ASY, Mekalanos JJ, Kranzusch PJ.
Cyclic dinucleotides (CDNs) have central roles in bacterial homeostasis and virulence by acting as nucleotide second messengers. Bacterial CDNs also elicit immune responses during infection when they are detected by pattern-recognition receptors in animal cells. Here we perform a systematic biochemical screen for bacterial signalling nucleotides and discover a large family of cGAS/DncV-like nucleotidyltransferases (CD-NTases) that use both purine and pyrimidine nucleotides to synthesize a diverse range of CDNs. A series of crystal structures establish CD-NTases as a structurally conserved family and reveal key contacts in the enzyme active-site lid that direct purine or pyrimidine selection. CD-NTase products are not restricted to CDNs and also include an unexpected class of cyclic trinucleotide compounds. Biochemical and cellular analyses of CD-NTase signalling nucleotides demonstrate that these cyclic di- and trinucleotides activate distinct host receptors and thus may modulate the interaction of both pathogens and commensal microbiota with their animal and plant hosts.
Nature. 2019 Mar;567(7747):194-199. doi: 10.1038/s41586-019-0953-5. Epub 2019 Feb 20.
Zhou W*, Whiteley AT*, de Oliveira Mann CC, Morehouse BR, Nowak RP, Fischer ES, Gray NS, Mekalanos JJ, Kranzusch PJ
*These authors contributed equally to this work
Cyclic GMP-AMP synthase (cGAS) recognition of cytosolic DNA is critical for immune responses to pathogen replication, cellular stress, and cancer. Existing structures of the mouse cGAS-DNA complex provide a model for enzyme activation but do not explain why human cGAS exhibits severely reduced levels of cyclic GMP-AMP (cGAMP) synthesis compared to other mammals. Here, we discover that enhanced DNA-length specificity restrains human cGAS activation. Using reconstitution of cGAMP signaling in bacteria, we mapped the determinant of human cGAS regulation to two amino acid substitutions in the DNA-binding surface. Human-specific substitutions are necessary and sufficient to direct preferential detection of long DNA. Crystal structures reveal why removal of human substitutions relaxes DNA-length specificity and explain how human-specific DNA interactions favor cGAS oligomerization. These results define how DNA-sensing in humans adapted for enhanced specificity and provide a model of the active human cGAS-DNA complex to enable structure-guided design of cGAS therapeutics.
Cell. 2018 Jul 12;174(2):300-311.e11. doi: 10.1016/j.cell.2018.06.026.
Whiteley AT, Garelis NE, Peterson BN, Choi PH, Tong L, Woodward JJ, Portnoy DA.
Cyclic diadenosine monophosphate (c-di-AMP) is a conserved nucleotide second messenger critical for bacterial growth and resistance to cell wall-active antibiotics. In Listeria monocytogenes, the sole diadenylate cyclase, DacA, is essential in rich, but not synthetic media and ΔdacA mutants are highly sensitive to the β-lactam antibiotic cefuroxime. In this study, loss of function mutations in the oligopeptide importer (oppABCDF) and glycine betaine importer (gbuABC) allowed ΔdacA mutants to grow in rich medium. Since oligopeptides were sufficient to inhibit growth of the ΔdacA mutant we hypothesized that oligopeptides act as osmolytes, similar to glycine betaine, to disrupt intracellular osmotic pressure. Supplementation with salt stabilized the ΔdacA mutant in rich medium and restored cefuroxime resistance. Additional suppressor mutations in the acetyl-CoA binding site of pyruvate carboxylase (PycA) rescued cefuroxime resistance and resulted in a 100-fold increase in virulence of the ΔdacA mutant. PycA is inhibited by c-di-AMP and these mutations prompted us to examine the role of TCA cycle enzymes. Inactivation of citrate synthase, but not down-stream enzymes suppressed ΔdacA phenotypes. These data suggested that c-di-AMP modulates central metabolism at the pyruvate node to moderate citrate production and indeed, the ΔdacA mutant accumulated six times the concentration of citrate present in wild-type bacteria.
Molecular Microbiology. April 2017;104(2):212-233. doi: 10.1111/mmi.13622. Epub 2017 Mar 8.

Whiteley AT, Ruhland BR, Edrozo MB, Reniere ML.
Bacterial pathogens have evolved sophisticated mechanisms to sense and adapt to redox stress in nature and within the host. However, deciphering the redox environment encountered by intracellular pathogens in the mammalian cytosol is challenging, and that environment remains poorly understood. In this study, we assessed the contributions of the two redox-responsive, Spx-family transcriptional regulators to the virulence of Listeria monocytogenes, a Gram-positive facultative intracellular pathogen. Spx-family proteins are highly conserved in Firmicutes, and the L. monocytogenes genome contains two paralogues, spxA1 and spxA2. Here, we demonstrate that spxA1, but not spxA2, is required for the oxidative stress response and pathogenesis. SpxA1 function appeared to be conserved with the Bacillus subtilis homologue, and resistance to oxidative stress required the canonical CXXC redox-sensing motif. Remarkably, spxA1 was essential for aerobic growth, demonstrating that L. monocytogenes SpxA1 likely regulates a distinct set of genes. Although the ΔspxA1 mutant did not grow in the presence of oxygen in the laboratory, it was able to replicate in macrophages and colonize the spleens, but not the livers, of infected mice. These data suggest that the redox state of bacteria during infection differs significantly from that of bacteria growing in vitro Further, the host cell cytosol may resemble an anaerobic environment, with tissue-specific variations in redox stress and oxygen concentration.
Infect Immunity. Apr 21, 2017; 85(5). pii: e00978-16. doi: 10.1128/IAI.00978-16. Print 2017 May.
Reniere ML*, Whiteley AT*, Portnoy DA.
*These authors contributed equally to this work
Listeria monocytogenes is an environmental saprophyte and facultative intracellular bacterial pathogen with a well-defined life-cycle that involves escape from a phagosome, rapid cytosolic growth, and ActA-dependent cell-to-cell spread, all of which are dependent on the master transcriptional regulator PrfA. The environmental cues that lead to temporal and spatial control of L. monocytogenes virulence gene expression are poorly understood. In this study, we took advantage of the robust up-regulation of ActA that occurs intracellularly and expressed Cre recombinase from the actA promoter and 5’ untranslated region in a strain in which loxP sites flanked essential genes, so that activation of actA led to bacterial death. Upon screening for transposon mutants that survived intracellularly, six genes were identified as necessary for ActA expression. Strikingly, most of the genes, including gshF, spxA1, yjbH, and ohrA, are predicted to play important roles in bacterial redox regulation. The mutants identified in the genetic selection fell into three broad categories: (1) those that failed to reach the cytosolic compartment; (2) mutants that entered the cytosol, but failed to activate the master virulence regulator PrfA; and (3) mutants that entered the cytosol and activated transcription of actA, but failed to synthesize it. The identification of mutants defective in vacuolar escape suggests that up-regulation of ActA occurs in the host cytosol and not the vacuole. Moreover, these results provide evidence for two non-redundant cytosolic cues; the first results in allosteric activation of PrfA via increased glutathione levels and transcriptional activation of actA while the second results in translational activation of actA and requires yjbH. Although the precise host cues have not yet been identified, we suggest that intracellular redox stress occurs as a consequence of both host and pathogen remodeling their metabolism upon infection.
PLoS Pathogens. July 14, 2016; 12(7):e1005741. doi: 10.1371/journal.ppat.1005741. eCollection 2016 Jul.
Whiteley AT, Pollock AJ, Portnoy DA.
Cyclic di-adenosine monophosphate (c-di-AMP) is a widely distributed second messenger that appears to be essential in multiple bacterial species, including the Gram-positive facultative intracellular pathogen Listeria monocytogenes. In this study, the only L. monocytogenes diadenylate cyclase gene, dacA, was deleted using a Cre-lox system activated during infection of cultured macrophages. All ΔdacA strains recovered from infected cells harbored one or more suppressor mutations that allowed growth in the absence of c-di-AMP. Suppressor mutations in the synthase domain of the bi-functional (p)ppGpp synthase/hydrolase led to reduced (p)ppGpp levels. A genetic assay confirmed that dacA was essential in wild-type but not strains lacking all three (p)ppGpp synthases. Further genetic analysis suggested that c-di-AMP was essential because accumulated (p)ppGpp altered GTP concentrations, thereby inactivating the pleiotropic transcriptional regulator CodY. We propose that c-di-AMP is conditionally essential for metabolic changes that occur in growth in rich medium and host cells but not minimal medium.
Cell Host & Microbe. June 10, 2015; 17(6):788-98. doi: 10.1016/j.chom.2015.05.006. Epub 2015 May 28.
Kellenberger CA, Chen C, Whiteley AT, Portnoy DA, Hammond MC.
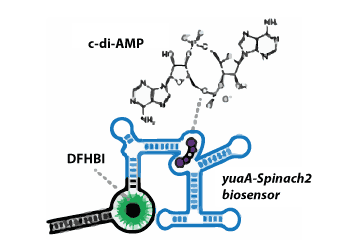
Cyclic di-AMP (cdiA) is a second messenger predicted to be widespread in Gram-positive bacteria, some Gram-negative bacteria, and Archaea. In the human pathogen Listeria monocytogenes, cdiA is an essential molecule that regulates metabolic function and cell wall homeostasis, and decreased levels of cdiA result in increased antibiotic susceptibility. We have generated fluorescent biosensors for cdiA through fusion of the Spinach2 aptamer to ligand-binding domains of cdiA riboswitches. The biosensor was used to visualize intracellular cdiA levels in live L. monocytogenes strains and to determine the catalytic domain of the phosphodiesterase PdeA. Furthermore, a flow cytometry assay based on this biosensor was used to screen for diadenylate cyclase activity and confirmed the enzymatic activity of DisA-like proteins from Clostridium difficile and Methanocaldococcus jannaschii. Thus, we have expanded the development of RNA-based biosensors for in vivo metabolite imaging in Gram-positive bacteria and have validated the first dinucleotide cyclase from Archaea.
Journal of the American Chemical Soc. May 27, 2015; 137(20):6432-5. doi: 10.1021/jacs.5b00275. Epub 2015 May 15.
Reniere ML, Whiteley AT, Hamilton KL, John SM, Lauer P, Brennan RG, Portnoy DA.
Intracellular pathogens are responsible for much of the world-wide morbidity and mortality due to infectious diseases. To colonize their hosts successfully, pathogens must sense their environment and regulate virulence gene expression appropriately. Accordingly, on entry into mammalian cells, the facultative intracellular bacterial pathogen Listeria monocytogenes remodels its transcriptional program by activating the master virulence regulator PrfA. Here we show that bacterial and host-derived glutathione are required to activate PrfA. In this study a genetic selection led to the identification of a bacterial mutant in glutathione synthase that exhibited reduced virulence gene expression and was attenuated 150-fold in mice. Genome sequencing of suppressor mutants that arose spontaneously in vivo revealed a single nucleotide change in prfA that locks the protein in the active conformation (PrfA*) and completely bypassed the requirement for glutathione during infection. Biochemical and genetic studies support a model in which glutathione-dependent PrfA activation is mediated by allosteric binding of glutathione to PrfA. Whereas glutathione and other low-molecular-weight thiols have important roles in redox homeostasis in all forms of life, here we demonstrate that glutathione represents a critical signalling molecule that activates the virulence of an intracellular pathogen.
Nature. January 8th, 2015; 517(7533):170-3. doi: 10.1038/nature14029.
Witte CE, Whiteley AT, Burke TP, Sauer JD, Portnoy DA, Woodward JJ.
Listeria monocytogenes infection leads to robust induction of an innate immune signaling pathway referred to as the cytosolic surveillance pathway (CSP), characterized by expression of beta interferon (IFN-β) and coregulated genes. We previously identified the IFN-β stimulatory ligand as secreted cyclic di-AMP. Synthesis of c-di-AMP in L. monocytogenes is catalyzed by the diadenylate cyclase DacA, and multidrug resistance transporters are necessary for secretion. To identify additional bacterial factors involved in L. monocytogenes detection by the CSP, we performed a forward genetic screen for mutants that induced altered levels of IFN-β. One mutant that stimulated elevated levels of IFN-β harbored a transposon insertion in the gene lmo0052. Lmo0052, renamed here PdeA, has homology to a cyclic di-AMP phosphodiesterase, GdpP (formerly YybT), of Bacillus subtilis and is able to degrade c-di-AMP to the linear dinucleotide pApA. Reduction of c-di-AMP levels by conditional depletion of the di-adenylate cyclase DacA or overexpression of PdeA led to marked decreases in growth rates, both in vitro and in macrophages. Additionally, mutants with altered levels of c-di-AMP had different susceptibilities to peptidoglycan-targeting antibiotics, suggesting that the molecule may be involved in regulating cell wall homeostasis. During intracellular infection, increases in c-di-AMP production led to hyperactivation of the CSP. Conditional depletion of dacA also led to increased IFN-β expression and a concomitant increase in host cell pyroptosis, a result of increased bacteriolysis and subsequent bacterial DNA release. These data suggest that c-di-AMP coordinates bacterial growth, cell wall stability, and responses to stress and plays a crucial role in the establishment of bacterial infection.
MBio. May 28, 2013; 4(3):e00282-13. doi: 10.1128/mBio.00282-13.